Our Approach
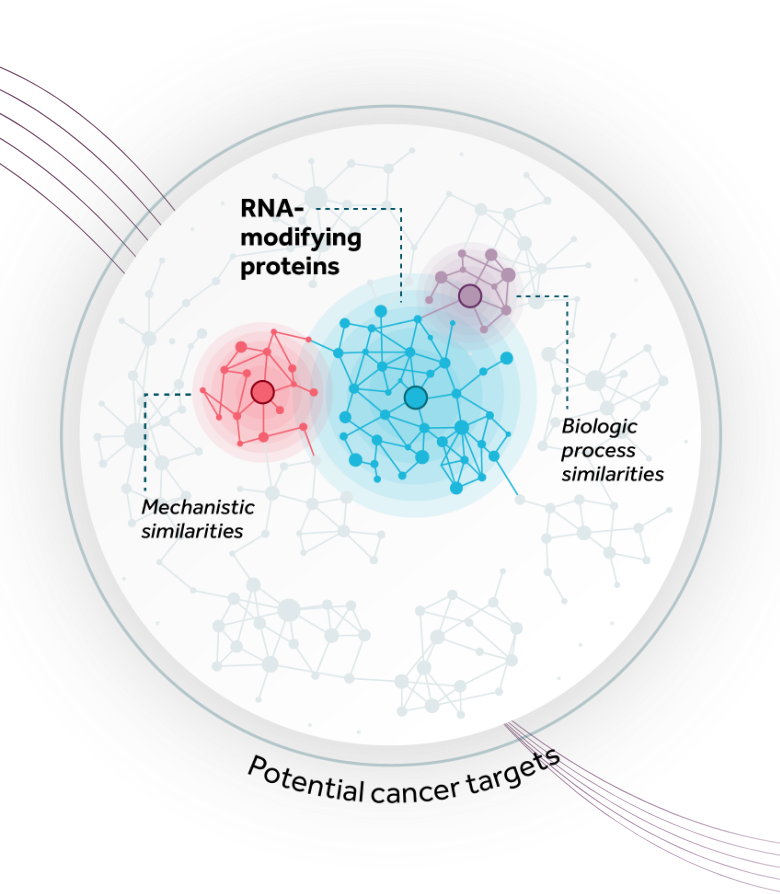
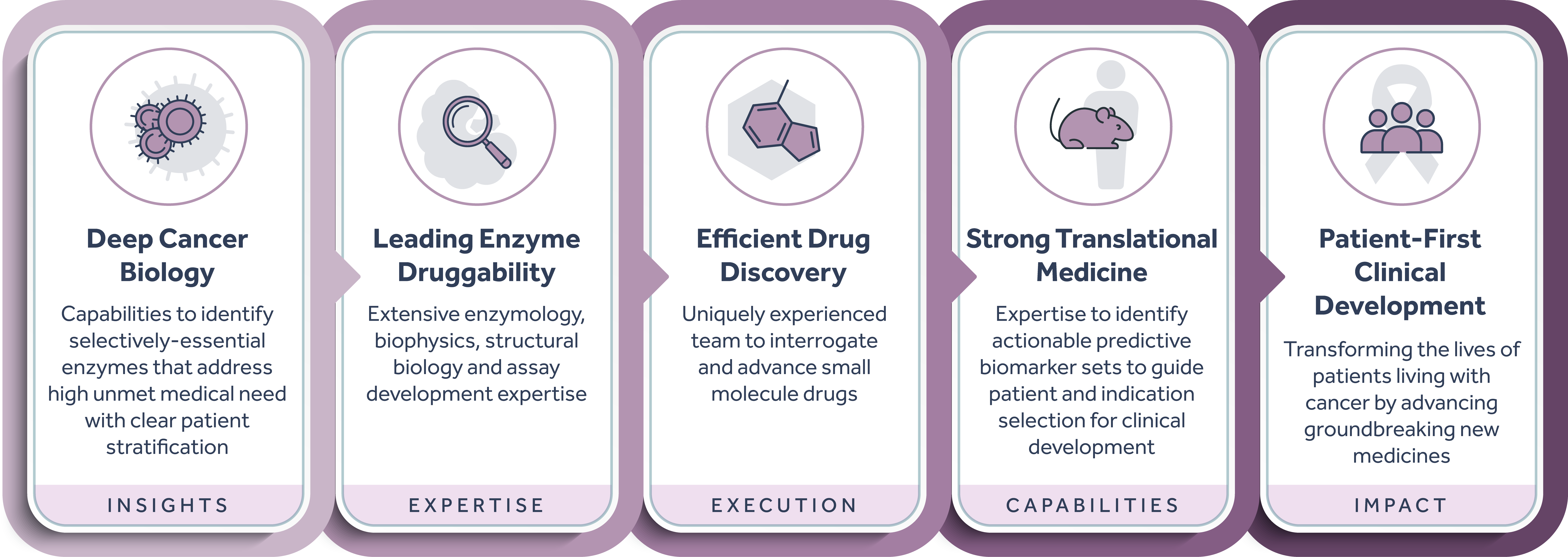
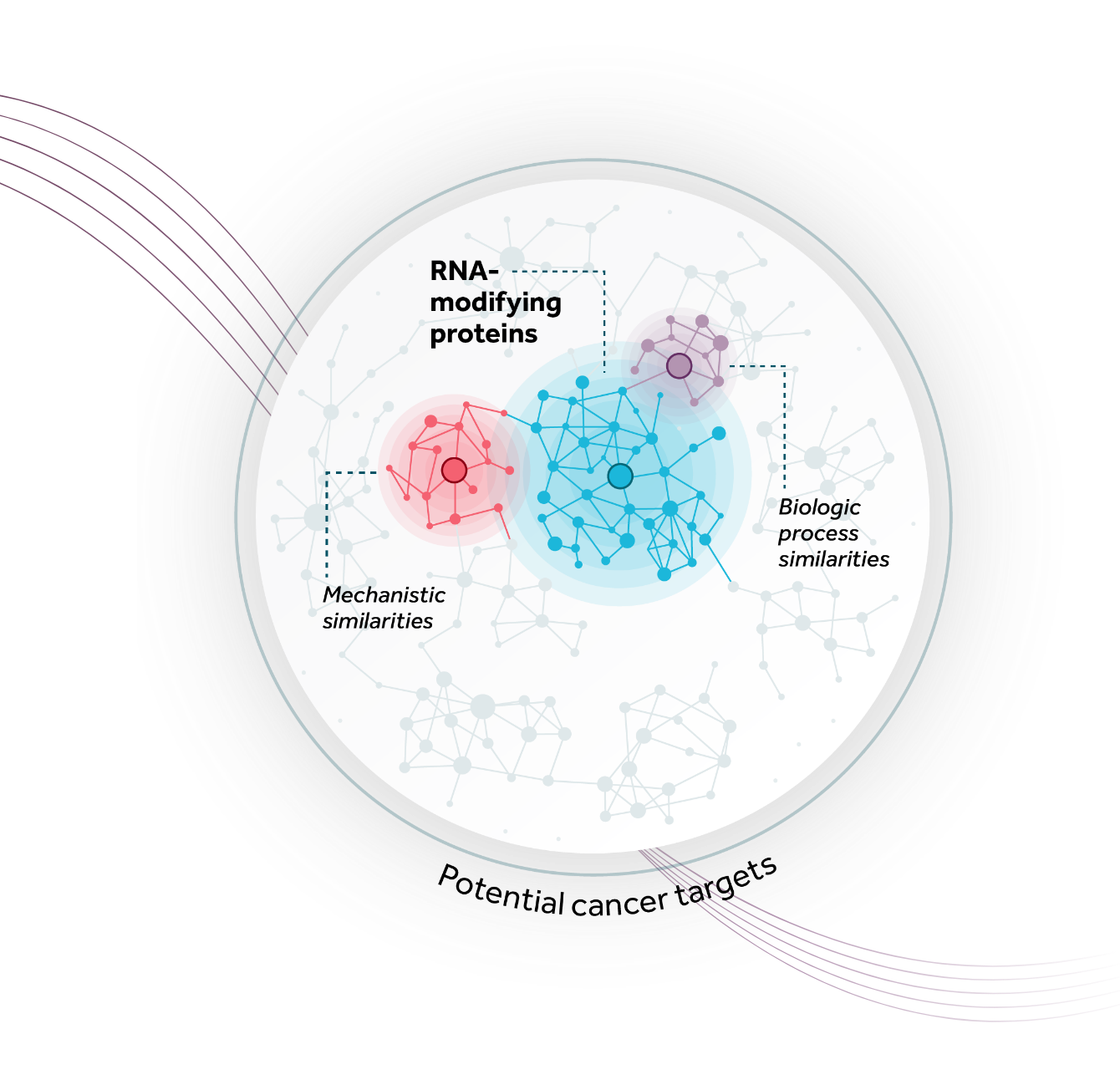
Our deep understanding of the key biological underpinnings of cancer and ability to identify, interrogate, and successfully drug intracellular enzyme targets enables us to advance a pipeline of unique small molecule therapeutics with expansive potential. Combining our industry-leading expertise in RNA-modifying proteins (RMPs) and our systematic mapping of both the RMP space and adjacent high-value areas for drug discovery, we have built a flexible model that allows for a diversity of approaches to developing potentially transformative biomarker-driven cancer medicines. Our therapies are designed for suboptimally-addressed, novel or known high-impact oncogenic targets with the potential to benefit large patient populations.